|
Introduction |
Cytosine methylation is one of the most important RNA epigenetic modifications. With the development of
experimental technology, scientists attach more importance to RNA cytosine methylation and find bisulfite
sequencing is an effective experimental method for RNA cytosine methylation study. However, rarely tools
can directly deal with RNA bisulfite sequencing data efficiently at present. Hence, we have developed a specialized
tool BS-RNA. Its annotation result is in BED (.bed) format, including locations, sequence context types
(CG/CHG/CHH, H = A, T, or C), reference
sequencing depths, cytosine sequencing depths, and methylation levels of covered cytosine sites on both Watson and
Crick strands. BS-RNA supports both paired-end and single-end sequencing short reads from a directional
bisulfite library. Evaluation results of the rates of uniquely mapped reads, rates of correctly mapped reads, and
running time for simulated data and actual data indicate that BS-RNA can provide fast and accurate mapping of
RNA bisulfite sequencing reads. Comparison between annotated cytosine methylation used BS-RNA and published
research also shows that BS-RNA is an effective annotation tool for RNA bisulfite sequencing data. |
Principle |
The BS-RNA process (Figure. 1-2) includes three main steps: pre-treatment, mapping, and annotation.
The first step
is the pre-treatment of reference genome sequences, sequencing data, and gene annotation file: (1) the reference
genome sequence is converted twice in parallel as follows: (A) cytosines are replaced by thymines and (B) guanines
are replaced by adenines. This genome sequence conversion only need to be performed the first time one uses the
reference genome sequence, which means it could be reused for all the following analysis which use the same reference
genome sequence. (2) cytosines in reads of the T-rich sequencing read file are replaced by thymines, while guanines
in reads of the A-rich sequencing read file are replaced by adenines. And (3) the gene annotation file in GTF format
is revised to fit the converted reference genome sequences. Each annotation line is converted twice simultaneously:
"C-T" and "G-A" are appended to the chromosome label in the gene annotation file, respectively.
Next, the HISAT2 program is invoked by BS-RNA to build alternative splicing according to the modified annotation gene
file and align pre-processed reads to the converted reference genome sequence. BS-RNA filters out two types of reads
that are mapped to the reference genome sequence as follows: (1) reads mapped to multiple positions and (2) reads
mapped to the wrong strands (T-rich reads mapped to reverse-complement of reference sequence converted C to T or to
reference sequence converted G to A, A-rich reads mapped to reference sequence converted C to T or to reverse
complements of reference sequence converted G to A). The original mapping file (SAM format), filtered mapping file
(SAM format), and mapping report file will be provided by BS-RNA when the mapping step is finished.
Further, BS-RNA splits the filtered mapping file into two files: a Watson-strand SAM format file (mapped to reference
converted C to T) and a Crick-strand SAM format file (mapped to reference converted G to A). SAMtools is embedded into
BS-RNA to convert the Watson-strand and Crick-strand SAM format files to BAM format and then sort these two BAM files
according to the chromosome coordination. Single-base coverage information is extracted using the mpileup command of
SAMtools from the sorted BAM files. For each cytosine position in the genome, the read base (SAMtools mpileup output
information) is deemed to support methylated cytosine sequencing if it matches a dot or a comma, otherwise it is deemed
to support an unmethylated cytosine sequencing. We excluded the reference skips (which menas "<" or ">" is not counted).
BS-RNA provides the annotation result for each covered cytosine in a BED (.bed) file with information related to the
methylation character: location of the covered cytosine in the reference, sequence context type (CG/CHG/CHH, H = A, T, or C),
reference sequencing depth (total number of reads mapped to the cytosine site), cytosine sequencing depth (total number of
reads that supported a methylated cytosine at this site), and methylation level (the ratio of cytosine sequencing depth to
sequencing depth at the cytosine site) on the Watson strand and Crick strands for each chromosome to the users. Annotation
files in the BED format make it easy to observe the methylation distributions using an IGV or UCSC browser.
|
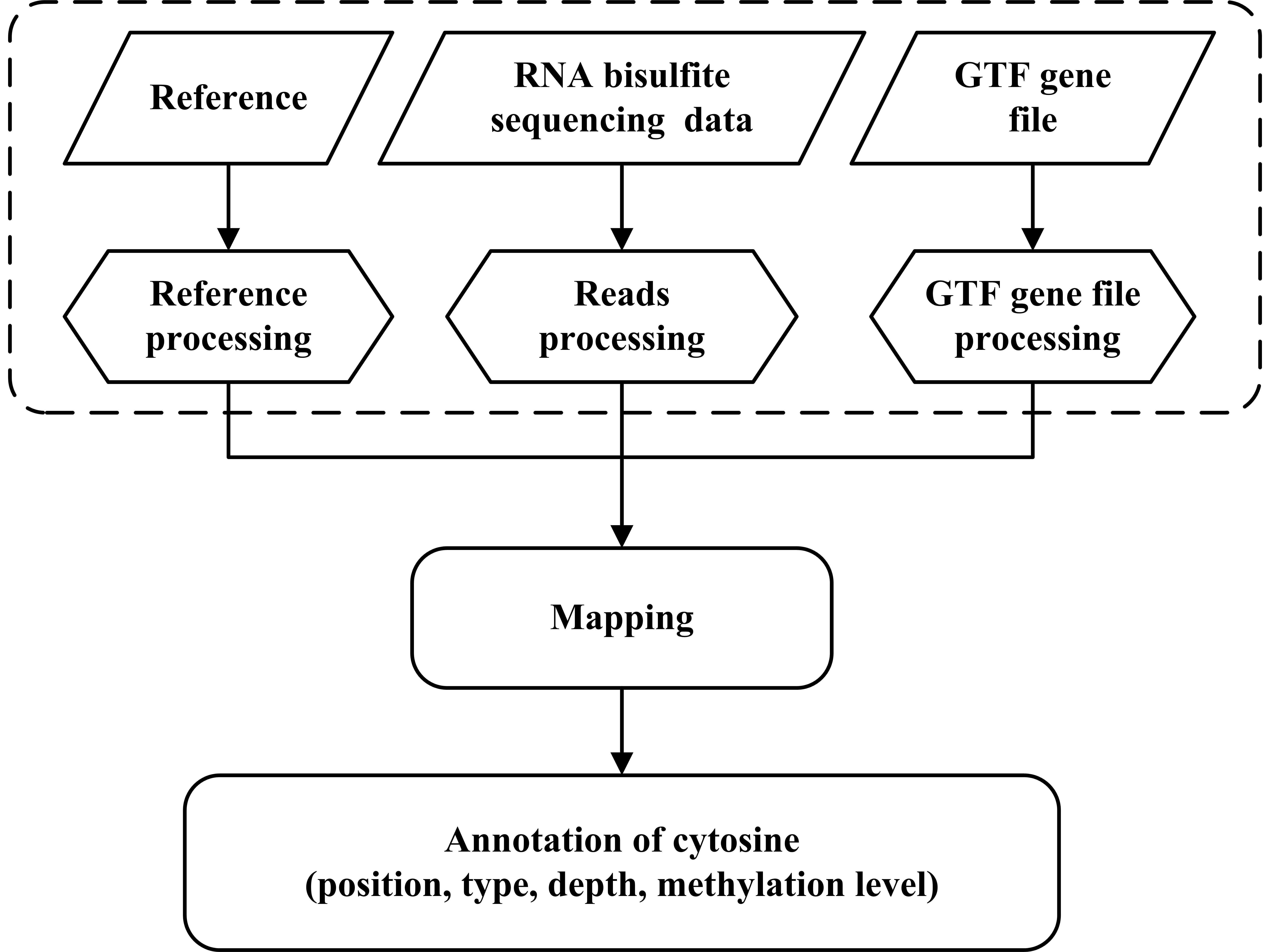 |
Fig.1. Flowchart of mapping and annotation of BS-RNA |
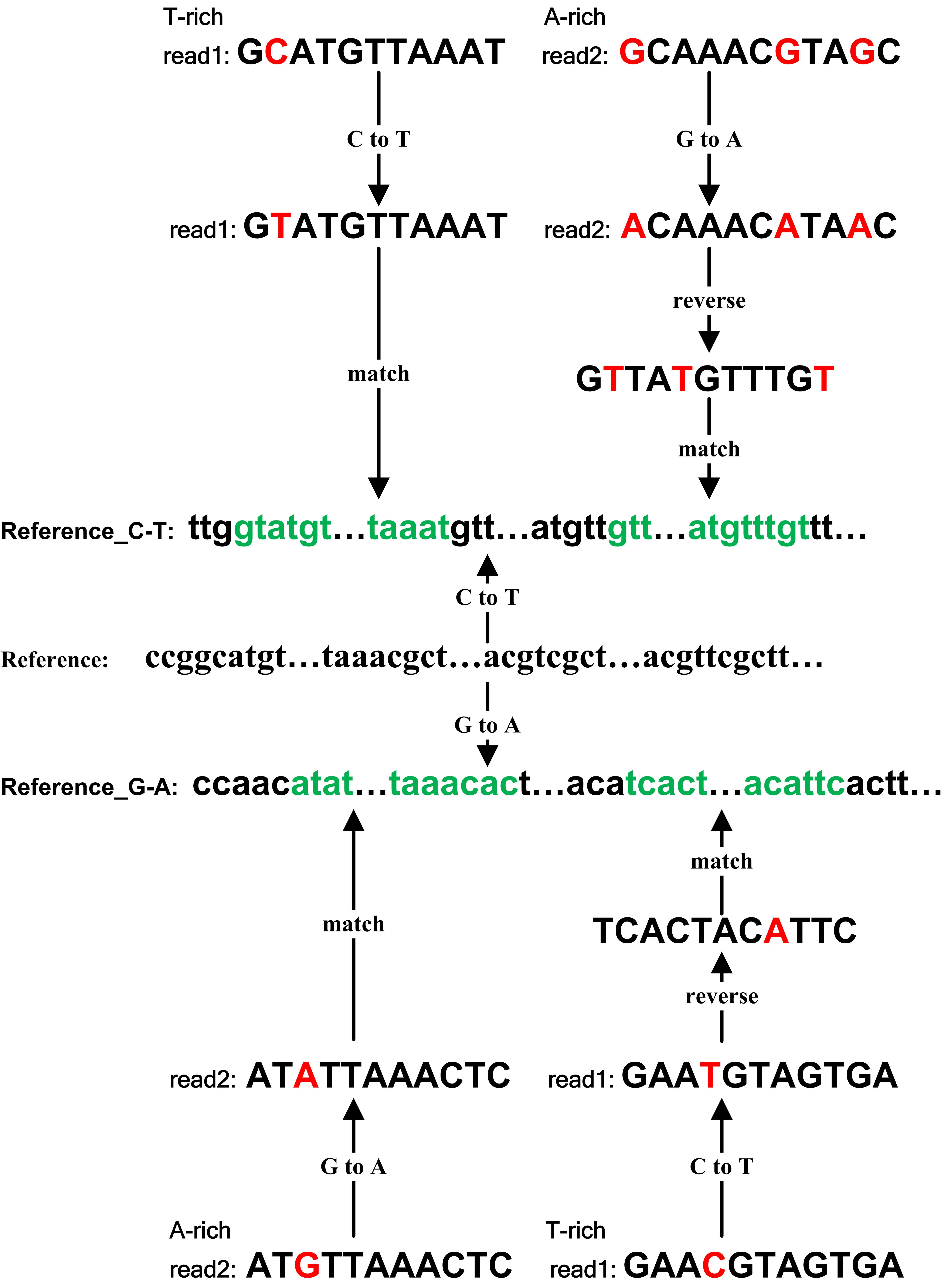 |
Fig.2. mapping principle of BS-RNA |
Downloads |
Source code of BS-RNA can be downloaded here for SAMtools-1.2 or older version.
Source code of BS-RNA can be downloaded here for SAMtools-1.3+ version.
To test the performance of BS-RNA, test sample data is provided here. The test data (simulated RNA bisulfite sequencing data set of human) is a paired-end dataset in FastQ format (Phred33). The length of read is 100bp. The command for test the demo data could be like this: |
perl BS-RNA_v1.0.pl --perlDir script --reads1 test_T-rich.fq --reads2 test_A-rich.fq --gene Homo_sapiens.GRCh37.75.gtf --rawRef hg19_ref --pathToPython /.../python2.7.8/bin --pathToHISAT2 /.../hisat2-2.0.1-beta
--pathToSAMtools /.../samtools-0.1.16 --outDir /.../demo_result |
It will take about 2.2 hours to complete all the analysis for this test data (including indexing the reference genome sequences) on a standalone, 2.4 GHz single-core processor with 13G memory. Please refer to the Manual for any questions. |
Installation |
BS-RNA is written in Perl and is executed from the command line in LINUX system. To install BS-RNA simply copy the BS-RNA_v1.0.tar.gz file (please download from http://bs-rna.big.ac.cn) into a BS-RNA installation folder and extract all the files by typing:
tar xzf BS-RNA_v1.0.tar.gz
BS-RNA requires a working of Perl, Python (at least Python2.7.8), HISAT2 (at least hisat2-2.0.1-beta) and SAMtools
(at least SAMtools-1.0). Therefore it is a requirement that they are installed on your machine. BS-RNA will
assume that these software are all in the working path unless their paths are specified manually. Furthermore
bowtie2 should also be in the working path as HISAT2 uses the bowtie2 implementation to handle most of the
operations on the FM index. |
|
Either paired-end or single-end reads with variable read length from strand-specific libraries are supported by BS-RNA.
The input sequence format should be uncompressed FastQ.
First you need download the reference genome sequences files of your concerned species and place them in a folder.
Only single-entry files are supported. BS-RNA supports reference genome sequences in FastA format. The name begin
with "chr" and the only allowed file extension is .fa. Secondly a gene model annotation file also need to be
downloaded, which should be in GTF format.
Furthermore, two configure files could be specified for indexing the reference genome sequences and mapping the RNA
sequencing data to the reference genome sequences if the user want to custom the corresponding parameters.
An instruction on how to generate the configure file for hisat2-build indexer or hisat2 could be found in the
downloaded package. Each option should be specified in one single line. |
| Usage: perl BS-RNA_v1.0.pl [Options] |
| Options: |
| --perlDir |
Path |
Full path of the perl scripts |
| --reads1 |
File |
Input T-rich reads file |
| --reads2 |
File |
Input A-rich reads file |
| --gene |
File |
Supply BS-RNA with a set of gene model annotations, a GTF format file |
| --rawRef |
Path |
Directory of raw reference genome sequences |
| --convertRef |
Path |
Directory of converted reference genome sequences |
| --pathToPython |
Path |
Full path </.../.../> to the Python installation on your system |
|
|
If not specified it is assumed that Python is in the PATH |
| --pathToHISAT2 |
Path |
Full path </.../.../> to the HISAT2 installation on your system |
|
|
If not specified it is assumed that HISAT2 is in the PATH |
| --pathToSAMtools |
Path |
Full path </.../.../> to the SAMtools installation on your system |
|
|
If not specified it is assumed that SAMtools is in the PATH |
| --phred64 |
|
Qualities are ASCII chars equal to the Phred quality plus 64 |
|
|
"off" means Qualities are ASCII chars equal to the Phred quality plus 33 |
|
|
"Default: off |
| --specBuild |
File |
Configure file for hisat2-build indexer |
| --specHisat2 |
File |
Configure file for HISAT2 |
| --outDir |
Path |
Result output directory |
| --h or help |
|
Display this message |
|
A typical command for analyzing paired-end RBS-seq data is as follows: |
perl BS-RNA_v1.0.pl --perlDir script --reads1 test_T-rich.fq --reads2 test_A-rich.fq --gene Homo_sapiens.GRCh37.75.gtf
--rawRef hg19_ref --phred64 --pathToPython /.../python2.7.8/bin
--pathToHISAT2 /.../hisat2-2.0.1-beta --pathToSAMtools /.../samtools-0.1.16 --outDir /.../demo_result |
While for a single-end T-rich reads file is like this: |
perl BS-RNA_v1.0.pl --perlDir script --reads1 test_T-rich.fq --gene Homo_sapiens.GRCh37.75.gtf --rawRef hg19_ref
--phred64 --pathToPython /.../python2.7.8/bin --pathToHISAT2
/.../hisat2-2.0.1-beta --pathToSAMtools /.../samtools-0.1.16 --outDir /.../demo_result |
Or for a single-end A-rich reads file: |
perl BS-RNA_v1.0.pl --perlDir script --reads2 test_A-rich.fq --gene Homo_sapiens.GRCh37.75.gtf --rawRef hg19_ref
--phred64 --pathToPython /.../python2.7.8/bin --pathToHISAT2
/.../hisat2-2.0.1-beta --pathToSAMtools /.../samtools-0.1.16 --outDir /.../demo_result |
|
If the reference genome sequences have been converted in the previous analysis, please skip this step by adding
this option to save time: "--convertRef path_of_converted_reference_genome". In this situation, BS-RNA
generates three folders in the specified output directory:
Map: contains mapping result file in SAM format and another file with spliced sites.
Filter: contains filtered mapping result file in SAM format and a statistic file called ˇ°filter_mapping.sam.maprateˇ± containing the following information:
|
| total: total reads number |
| map: mapped reads number |
| uniq: uniq mapped reads number |
| cor: correctly mapping on a corresponding strand reads number |
| used%: percent of correctly mapping on a corresponding strand reads |
|
ps. The reads are mapped to the converted reference genome sequences, therefore the chromosome present in the
SAM file contain "C-T" (represent the chromosome which convert all cytosines to thymines) or "G-A" (represent the chromosome which convert all guanines to adenines). |
Level: contains bed files, which presents the following information for each covered cytosine site: |
| chr: name of the chromosome |
| start: cytosine chromosomal coordinates (0-based) |
| end: cytosine chromosomal coordinates (1-based) |
| strand: "+" means forward strand and "-" means crick strand |
| mCtype: methylation site type, one of the following [CG, CHG, CHH] |
| depth: total number of reads mapped to the cytosine site |
| mCdep: total number of reads that supported a methylated cytosine at this position |
| level: methylation level at the cytosine position |
|
If the "--convertRef" option is not specified, an extra folder named "ref_C-T_G-A" will also be created in the
output directory. This folder contains the concatenated raw genomce sequences and converted genome sequences in
FastA format as well as the corresponding bowtie2 indexed files. |
|
|
|

